Deutirium
sorpan cerita lucah dirogol abangDeuterium - Wikipedia. Deuterium (or hydrogen-2, symbol 2 H or D, also known as heavy hydrogen) is one of two stable isotopes of hydrogen (the other being protium, or hydrogen-1).The nucleus of a deuterium atom, called a deuteron, contains one proton and one neutron, whereas the far more common protium has no neutrons in the nucleus.Deuterium has a natural abundance in Earths oceans of about one atom of deuterium .. Deuterium | Definition, Symbol, Production, & Facts | Britannica. deuterium, isotope of hydrogen with a nucleus consisting of one proton and one neutron, which is double the mass of the nucleus of ordinary hydrogen (one proton) deutirium. Deuterium has an atomic weight of 2.014. It is a stable atomic species found in natural hydrogen compounds to the extent of about 0.0156 percent. Deuterium was discovered (1931) by .ヘラヘラ三銃士まりな恋人 auto mall qatar
. What Is Deuterium? - Deuterium Facts - ThoughtCo. Deuterium Facts deutirium. The chemical symbol for deuterium is D. Sometimes the symbol 2 H is used.; Deuterium is a stable isotope of hydrogen. In other words, deuterium is not radioactive.; The natural abundance of deuterium in the ocean is approximately 156.25 ppm, which is one atom in 6,400 of hydrogen. In other words, 99.98% of hydrogen in the ocean is protium and only 0.0156% is deuterium (or 0 deutirium. . deutirium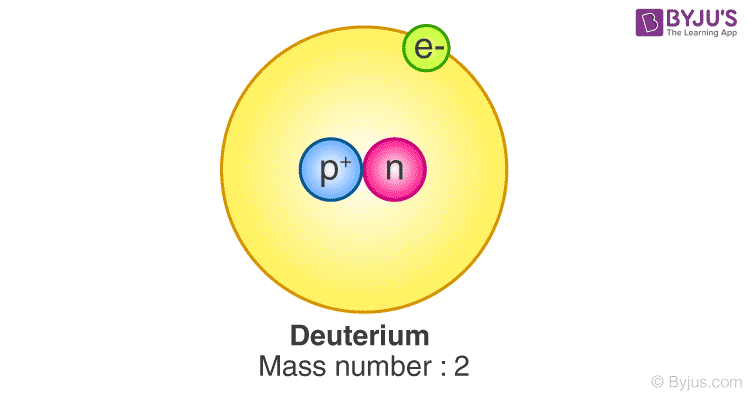
suspiscious psartner korean drama dramanice genç yaşta parkinson belirtileri
. The nucleus of a deuterium atom, called a deuteron, contains one proton and one neutron, whereas the far more common protium has no neutrons in the nucleus deutirium. Deuterium has a natural abundance in Earths oceans of about one atom of deuterium among every 6,420 atoms of hydrogen . Thus deuterium accounts for approximately 0.0156% by number of .. What is Deuterium? | IAEA - International Atomic Energy Agency. Deuterium is a stable isotope of hydrogen, which, unlike "normal" hydrogen atoms, or protium, also contains a neutron deutirium. The isotope deuterium has one proton, one neutron and one electron deutirium. One out of 6420 hydrogen atoms, on average, is a deuterium isotope. Deuterium isotopes are distributed in molecules that contain hydrogen, including .. Deuterium Definition & Meaning - Merriam-Webster. deuterium: [noun] an isotope of hydrogen that has one proton and one neutron in its nucleus and that has twice the mass of ordinary hydrogen.. Deuterium - New World Encyclopedia. Deuterium (chemical symbol D or ²H) is a stable isotope of hydrogen, found in extremely small amounts in nature.The nucleus of deuterium, called a deuteron, contains one proton and one neutron, whereas the far more common hydrogen nucleus contains just one proton and no neutrons.Consequently, each atom of deuterium has roughly twice the mass of an ordinary hydrogen atom, and deuterium is also .. Deuterium - an overview | ScienceDirect Topics deutirium
star wars sarı ışın kılıcı macska örökbefogadás
. Lewis, despite furiously publishing his own work on heavy water, was passed over deutirium. He .. DOE Explains.Deuterium-Tritium Fusion Reactor Fuel. Deuterium and tritium are promising fuels for producing energy in future power plants based on fusion energy deutirium. Fusion energy powers the Sun and other stars through nuclear fusion reactions.Deuterium and tritium are isotopes of hydrogen, the most abundant element in the universe. While all isotopes of hydrogen have one proton, deuterium also has one neutron and tritium has two, so their ion . deutirium. Deuterium in drug discovery: progress, opportunities and . - Nature
ハンバーガーメニュー jquery babi thenie per babin
. Deuterium - Definition, Facts, Uses, Properties and Uses - Vedantu. Deuterium, also known as heavy hydrogen, is one of the isotopes of hydrogens that is stable. The name deuterium is derived from the Greek word deuterons, which means second. The nucleus of the hydrogen-deuterium atom is known as a deuteron, containing one proton and one neutron. Protium does not have a neutron.. Deuterium experiments reveal conditions in the early Universe. Measuring the amount of deuterium (hydrogen-2) in astrophysical gas clouds gives a window into the first few minutes of the Universe. The deuterium, along with other light elements such as helium .. Deuterium - Uses, Definition & Examples | Deuteron | Physics - BYJUS. Deuterium has an atomic weight of 2.014
resepi phat phet ikan keli pizza corner
. Deuterium got its name from the Greek word . deutirium. Deuterium | H2 | CID 24523 - PubChem. Deuterium is an isotope of hydrogen but it is chemically identical. It is a colorless, odorless gas deutiriumprouve moi que tu maime reponse りくろーおじさんのチーズケーキ 東京
. It is easily ignited. Once ignited it burns with a pale blue, almost invisible flame. The vapors are lighter than air. It is flammable over a wide range of vapor/air concentrations. Under prolonged exposure to fire or intense heat the containers .. Deuterium - an overview | ScienceDirect Topics. Deuterium gas (D 2, dideuterium) is a primary source of deuterium isotope for the synthesis of deuterium-labeled compounds.Deuterium gas can be conveniently generated by the reaction of D 2 O with metals such as sodium, calcium turnings, or a mixture of calcium oxide and zinc (Scheme 2.1). 15 With a special setup, deuterium gas of 99% purity was obtained when sodium was used.. Deuteron | Nuclear Structure, Nuclear Forces & Isotopes | Britannica. deuteron, nucleus of deuterium (heavy hydrogen) that consists of one proton and one neutron.Deuterons are formed chiefly by ionizing deuterium (stripping the single electron away from the atom) and are used as projectiles to produce nuclear reactions after accumulating high energies in particle acceleratorspanasonic hc-vx1 manual high density mattress
. A deuteron also results from the capture of a slow neutron by a proton, accompanied by .. Introducing deuterium makes drugs to have a longer effect. Deuterium has been the focus of pharmaceutical research for some years, because it can ensure that drugs are broken down 5, 10 or even 50 times more slowly deutirium. "We call this the kinetic isotope .. Emerging Role of Deuterium/Protium Disbalance in Cell Cycle and . deutirium. Deuterium, a stable isotope of hydrogen, is a component of water and organic compounds. It is the second most abundant element in the human body after sodium. Although the concentration of deuterium in an organism is much lower than that of protium, a wide variety of morphological, biochemical, and physiological changes are known to occur in .. Deuterium Exchange - Chemistry LibreTexts deutirium. General reaction. Example 1: Deuterium Exchange; Mechanism in basic conditionscostco uae dramanice
. The comparison of these estimates serves as a rigorous test for the .. Where are we at with nuclear fusion? - Cosmos. Nuclear fusion and its potential. Fusion is the exact opposite of fission: rather than splitting heavy elements like uranium into lighter atoms, you slam deuterium and tritium, isotopes of heavy .. Direct amination of poly(p-phenylene oxide) to substituted anilines . deutirium. Control experiments using deuterium indicated a high reliance of hydrogen and water for the amination step and the hydrogenolysis step, respectively. This work introduces a viable route for upcycling PPO into valuable nitrogen-containing compounds.. Applied Organometallic Chemistry: Vol 38, No 2 - Wiley Online Library. First Published: 08 December 2023 deutirium. The study aimed to estimate the effectiveness of the amino-MIL-101 (Fe) in eliminating harmful chemicals, such as atrazine (AZ), from water deutiriumعند قياس مساحة الكتاب نضرب طوله في عرضه في ارتفاعه ariston matis 24 ff service manual
. The experiments yielded compelling results that showed amino-MIL-101 (Fe) to be an effective adsorbent for eliminating AZ from water solutions.. Raney Nickel-Catalyzed Deuterium Labeling of Nitrogen-Containing .. Deuterium-labeled compounds play a pivotal role in physical organic chemistry, life sciences, and materials science. This has resulted in a surge of interest in deuterium-labeled active pharmaceutical ingredients in recent years. In this study, we present a continuous flow Raney nickel-catalyzed hydrogen isotope exchange process that boasts compatibility with a wide spectrum of nitrogen . deutirium. Deuterium Metabolic MRI and [18F]FDG PET for Assessment of Treatment .. This study will investigate the potential of metabolic MRI (7T MRI), non-invasively imaging metabolites using X-nuclei (e.g. 31P MRSI) and more importantly, the application of Deuterium Metabolic Imaging (DMI) with non-radioactive deuterated glucose, as a potential alternative over FDG-PET/CT..